Lithium-ion Batteries: Fire Risks and Loss Prevention in Yachting (part I)

1) Introduction/Background
2) Lithium-ion (Li-ion) Batteries
3) Hazards Associated with Li-ion Batteries, their Causes, and Consequences
4) Loss Prevention Guidance
5) Fire Suppression
6) Summary
References
1) Introduction/Background
There has been a steep increase in the number of fires on super yachts. It is estimated that between February 2018 until November 2023 more than 50 super yachts have been completely damaged by fire. Whilst the causes of some of these fires are known and have nothing to do with Li-ion batteries, others remain unexplained. A potential explanation could be Li-ion batteries, one of the issues in determining the cause of the fire is that the intensity of the flames is such that yachts turn into a fire ball and nothing is left after such fires! As per Heike Deg-gim, quoted by FT, head of safety at the UN’s Inter¬na¬tional Mari¬time Organ¬iz¬a-tion, said: “Lith¬ium bat¬ter¬ies have been recognized as poten¬tially haz¬ard¬ous when it comes to fire risk”.
There are certain cases where it was almost established that the cause of the fire was Lithium-ion batteries. This was the case for M/Y Kanga (40.88 m., GT 497, YOB 2018) said to be insured for 18 million Euros. A very interesting safety investigation report from the Marine Safety Investigation Unit of Transport Malta concluded that in “all probability” the seat of the fire was Lithium-ion batteries. What makes it creepy is that according to the crew none of the batteries were being recharged at the time of the fire, in the garage where the damaged batteries were stored the extraction fan was running.
The efficiency and high energy of Li-ion batteries have made them the backbone of every technology that needs battery support, from our daily mobile phones to electrical vehicles and even space stations. The decarbonization paradigm is such that increasingly toys and tenders stored and charged on superyachts are electrically powered by using Li-ion batteries such as e-scooters, sea bobs, electric jet skis, e-foils, electric tenders etc. This shift has occurred, whilst no really serious consideration was given to fire prevention or early detection measures to be taken to ensure that these measures which were valid for petrol-fueled crafts are still suitable for this new generation of e-powered crafts. This is even more intriguing, especially that risks associated with the charging of e-toys/tenders, to be efficiently mitigated should be integrated at an early stage of design/construction.
2) Lithium-ion (Li-ion) Batteries
Typically, batteries all work alike: a stream of electrically charged atoms known as ions flow through an electrolyte from the anode to the cathode, the cell’s two electrodes to generate a current.
The below figures (1, 2 & 4) have been extracted from Brookes Bell Lithium batteries whitepaper (*).
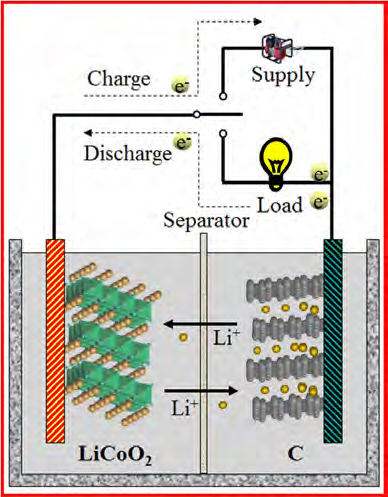
Li-ion batteries are usually safe and stable; however, they are extremely energy dense. They can store up to four times more energy than other batteries. As we all know, the bigger the amount of energy stored in a battery, the higher is the potential risk and severity (**). The speedy technology improvement is heightening the battery energy density in a way which makes it difficult for best design, construction methods, risk management and safety measures to cope with.
Li-ion battery cells combine highly flammable electrolytes, oxygen-rich cathodes, flammable gases with significant stored energy. Electrolytes consists of organic solvents and lithium salt. The organic solvents are the main cause of fires which are fueled by the oxygen present in the cathodes. This oxygen can be released if specific incidents occur like internal short circuits or excessive heats etc. As you know, batteries due to their chemical path have their own intrinsic internal resistance. This resistance is concomitant with the electrical current, when there is current which is circulating, the internal resistance heats up. This resistance can be mitigated by using thin large plates; but there are always limitations to what you can do.
Li-ion batteries contain flammable/toxic gases such as hydrogen (H2), carbon monoxide (CO), methane (CH4) and hydrofluoric acid (HF). Their release can cause fire or explosion or even intoxicate humans.
In order to boost energy or to increase the current or voltage, Li-ion batteries are usually constructed of connected cells. These connected cells are known as a module which are incorporated with other modules and components to form a battery pack. Please refer to the below figure:
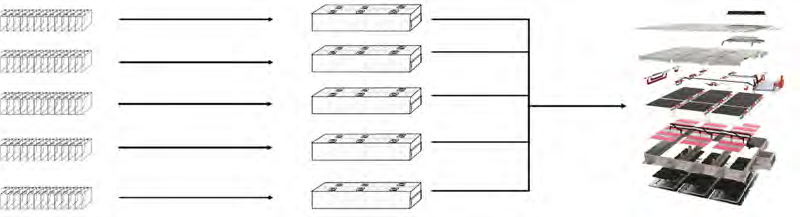
3) Hazards Associated with Li-ion Batteries, their Causes, and Consequences
a) Hazards (**)
a-1) Fire or Thermal runaway
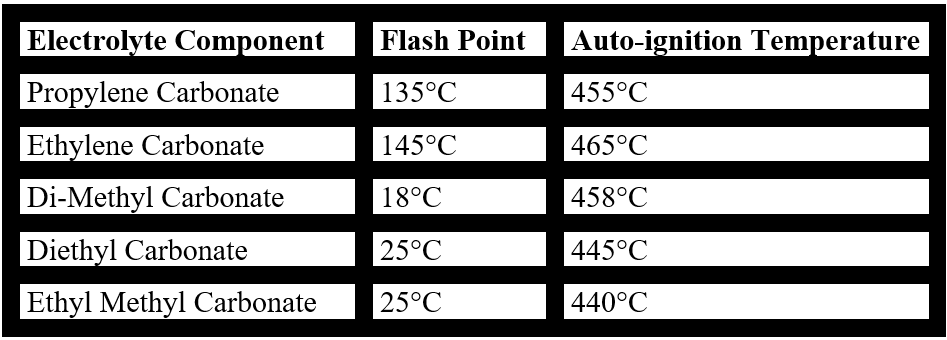
As we saw above, a Li-ion battery can be found always an electrolyte which is highly flammable, electrolytes contain commonly Li-ion cells with very low flash points (FP) like Di-Methyl Carbonate (FP = 180C), Diethyl Carbonate (FP = 250C) and Ethyl Methyl Carbonate (FP = 250C). FP is the temperature at which a particular organic compound gives off sufficient vapor to ignite in air. The electrolyte is not the only battery component which is combustible. The anode material is also combustible when the battery is highly charged. Under certain circumstances (like a short-circuit for example) if the heat generated by the short-circuit is unable to dissipate due to being in a closed environment, the temperature will rise quickly to a point of ignition. This fire will spread quickly from a cell to adjacent cells and then from the module to neighboring ones. It is almost inevitable that the fire turns into a thermal runaway fire which is self-sustainable. The battery cell temperature rises incredibly fast (milliseconds). The energy stored in that battery is released very suddenly. This chain reaction creates extremely high temperatures (around 400 degrees Celsius).
Table 3: Measured Flash Points and Auto-ignition Temperatures of some typical Lithium-ion cell electrolyte components
One more feature of Lithium batteries fire is that they burn for long periods and can rekindle hours, days or even weeks later and this can happen many times.
a-2) Explosion
If the ignitable vapor is released in a confined space (like a closed package, a non-ventilated garage or an intermodal freight container) there is a high risk of an explosion.
a-3) Toxic gases
The fire or explosion can release corrosive, irritating or poisonous gases.
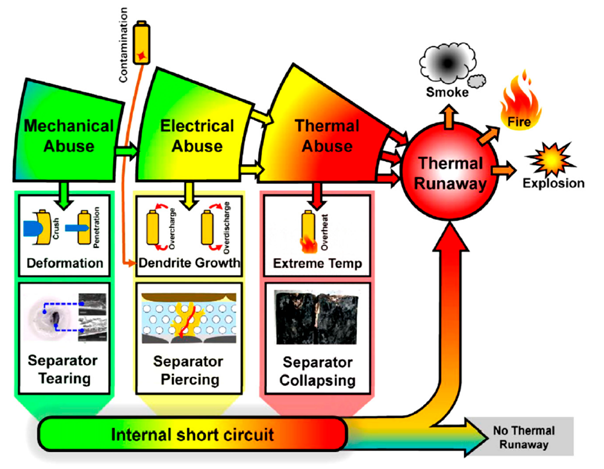
b) Causes (*)
The causes of hazards can be categorized into three types: mechanical, electrical and thermal abuse.
b-1) Mechanical Abuse
This is an external physical damage to the casing of the Li-ion battery like indentation or punctures. The deformation of the casing could lead for example to the breakages of the separator or the current collector enabling electrodes to come into contact, resulting in a short circuit.
This is what has happened on M/Y Kanga. It was documented that before catching fire, three of the four battery packs of the electric surfboards were experiencing sea water leakage. Seafarers had noticed brownish-colored water leaking from the inside of the batteries, and they intended to return them for repair/replacement. The manufacturers advised the crew not to use them or charge them and to check the internal status of the battery packs before sending them back.
After a damage to the battery casing, air may enter, react with the organic solution (electrolyte) and create heat.
b-2) Electrical Abuse
This may happen when overcharging or over-discharging the battery; they both have similar effects leading to instability.
Overcharging batteries may decompose the electrolyte on the cathode surface, thus increasing the battery temperature, releasing the cathodes of the oxygen in heat generating side reactions. These reactions can cause the battery rupture. There are as well excess Li-ions depositing on the anode to form lithium dendrites which are metallic branch-like structures that grow on the negative electrode during charging. These dendrites by piercing the separator can trigger a short circuit with all the dire consequences it may entail.
During over-discharge, a continuous release of Li-ions from the anode can oxidize the copper current collector which releases in turn copper ions. These copper ions can deposit on the cathode surface and trigger a short-circuit.
Similarly, when a Li-ion battery is discharged completely (when stored or not used for a long time), then both the cathode and anode begin to break down. The copper of the anode current collector will start to dissolve into the electrolyte. The copper ions start to precipitate into metallic copper that may lead to a short circuit when the battery is again charged.
Charging a Li-ion battery in cold temperatures (below 0oC) can cause lithium plating which cannot be removed. One this occurs a high-rate charging can damage the battery and cause a short-circuit, or it can be more easily damaged by a physical impact.
b-3) Thermal Abuse
Extreme temperatures can be either external temperature or temperature inside the battery itself. In principle, the battery cycling should not lead to safety accidents since the heat produced during normal use should not be enough to cause a big increase in temperature. However, there are certain cases where heat accumulates rather than dissipates since heat release rate often exceeds the cooling rate. This accumulation can lead to side reactions. “600C is the critical temperature, above which Li-ion batteries are prone to fail (*)”.
Temperatures in multi modal container can reach the double of the ambient temperatures. In the middle east, the ambient temperature can easily reach during the summer 400C to 500C, and the double exceeds easily the critical temperature mentioned above. We don’t have to forget that charging especially in a confined space produce more heat.
“Thermal stress or shock can result in a build-up of pressure, which may eventually lead to an explosion” (*).
c) Consequences of Abuse (*)
As we saw, Lithium-ion battery cells combine a flammable electrolyte with significant stored energy and oxygen. If a lithium-ion battery cell creates more heat than it can effectively disperse, it can lead to a rapid uncontrolled release of heat energy, known as ‘thermal runaway’, that can result in a fire or explosion.
Temperatures in lithium-ion battery cells can rise very quickly so that there is no time to react.